1. As a follow-up to the MyFunding Downtime Alert 1/21/2022, the issue impacting the Study Record(s) section of the PHS Human Subjects and Clinical Trials Information V3.0 form on the SF424 has been resolved.
2. Please see the following tips for using the current version of the SF424 upgrade:
Tip No. 1
If the response to the Primary Purpose question on the PHS Human Subjects and Clinical Trials Information V3.0 form (FORMS-G) is ‘other’, please prepare and submit proposal via NIH ASSIST or Grants.gov Workspace. Otherwise, an error will be generated (see Figures 1 & 2).
Figure 1. PHS Human Subjects and Clinical Trials Information V3.0 form
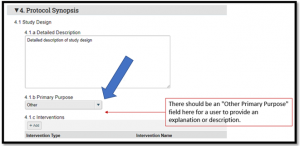 Figure 2. Error
Figure 2. Error
 Please note: This issue only impacts submissions where the Primary Purpose selected on a Study Record on the PHS Human Subjects and Clinical Trials Information V3.0 form is 'Other'. If the primary purpose is NOT “Other”, continue to submit System-to-System (i.e., via Grants.Gov) via MyFunding.
Tip No. 2
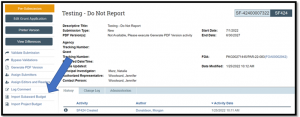
Use the ‘Import Subaward Budget’ activity in the SF424 Workspace to use the R&R Subaward Budget Form V3.0 form for FORMS-G.
Figure 3.
Please note: This issue only impacts submissions where the Primary Purpose selected on a Study Record on the PHS Human Subjects and Clinical Trials Information V3.0 form is 'Other'. If the primary purpose is NOT “Other”, continue to submit System-to-System (i.e., via Grants.Gov) via MyFunding.
Tip No. 2
Use the ‘Import Subaward Budget’ activity in the SF424 Workspace to use the R&R Subaward Budget Form V3.0 form for FORMS-G.
Figure 3.
 Tip No. 3
To use the SF424 Subaward Import activity in FORMS G, use the R&R Budget V1.4 form to import the data into the SF424 application. Otherwise, an error will be generated (see Figures 4).
Figure 4.
Tip No. 3
To use the SF424 Subaward Import activity in FORMS G, use the R&R Budget V1.4 form to import the data into the SF424 application. Otherwise, an error will be generated (see Figures 4).
Figure 4.
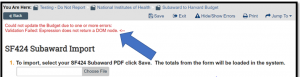
 Figure 2. Error
Figure 2. Error
 Please note: This issue only impacts submissions where the Primary Purpose selected on a Study Record on the PHS Human Subjects and Clinical Trials Information V3.0 form is 'Other'. If the primary purpose is NOT “Other”, continue to submit System-to-System (i.e., via Grants.Gov) via MyFunding.
Tip No. 2
Use the ‘Import Subaward Budget’ activity in the SF424 Workspace to use the R&R Subaward Budget Form V3.0 form for FORMS-G.
Figure 3.
Please note: This issue only impacts submissions where the Primary Purpose selected on a Study Record on the PHS Human Subjects and Clinical Trials Information V3.0 form is 'Other'. If the primary purpose is NOT “Other”, continue to submit System-to-System (i.e., via Grants.Gov) via MyFunding.
Tip No. 2
Use the ‘Import Subaward Budget’ activity in the SF424 Workspace to use the R&R Subaward Budget Form V3.0 form for FORMS-G.
Figure 3.
 Tip No. 3
To use the SF424 Subaward Import activity in FORMS G, use the R&R Budget V1.4 form to import the data into the SF424 application. Otherwise, an error will be generated (see Figures 4).
Figure 4.
Tip No. 3
To use the SF424 Subaward Import activity in FORMS G, use the R&R Budget V1.4 form to import the data into the SF424 application. Otherwise, an error will be generated (see Figures 4).
Figure 4.

News Categories
