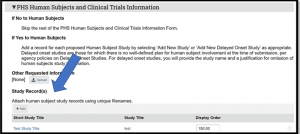
As a follow-up to the SF424 MyFunding Upgrade notice below, a vendor issue is impacting the Study Record(s) section of the PHS Human Subjects and Clinical Trials Information V3.0 form on the SF424 (see Figure 1).
Figure 1. PHS Human Subjects and Clinical Trials Information V3.0 page example
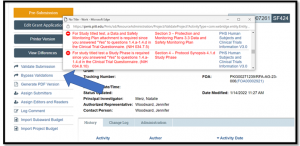
 Clicking the ‘Validate Submission’ activity will generate an error message (see Figure 2). DO NOT USE THE ‘BYPASS VALIDATIONS’ ACTIVITY TO WORK AROUND THE ERROR AND SUBMIT THE PROPOSAL (see Figure 3).
For proposals that require the PHS Human Subjects and Clinical Trials Information V3.0 form, please prepare and submit via Grants.gov Workspace or NIH ASSIST.
Figure 2. Error Message
Clicking the ‘Validate Submission’ activity will generate an error message (see Figure 2). DO NOT USE THE ‘BYPASS VALIDATIONS’ ACTIVITY TO WORK AROUND THE ERROR AND SUBMIT THE PROPOSAL (see Figure 3).
For proposals that require the PHS Human Subjects and Clinical Trials Information V3.0 form, please prepare and submit via Grants.gov Workspace or NIH ASSIST.
Figure 2. Error Message
 Figure 3. SF424 Workspace
Figure 3. SF424 Workspace
 PLEASE NOTE: This issue does not impact submissions that do not include a Study Record on the PHS Human Subjects and Clinical Trials Information V3.0 form.
The PERIS™ team is working with our vendor to resolve this issue as quickly as possible and will alert users once this has been corrected. Thank you for your patience.
PLEASE NOTE: This issue does not impact submissions that do not include a Study Record on the PHS Human Subjects and Clinical Trials Information V3.0 form.
The PERIS™ team is working with our vendor to resolve this issue as quickly as possible and will alert users once this has been corrected. Thank you for your patience.
 Clicking the ‘Validate Submission’ activity will generate an error message (see Figure 2). DO NOT USE THE ‘BYPASS VALIDATIONS’ ACTIVITY TO WORK AROUND THE ERROR AND SUBMIT THE PROPOSAL (see Figure 3).
For proposals that require the PHS Human Subjects and Clinical Trials Information V3.0 form, please prepare and submit via Grants.gov Workspace or NIH ASSIST.
Figure 2. Error Message
Clicking the ‘Validate Submission’ activity will generate an error message (see Figure 2). DO NOT USE THE ‘BYPASS VALIDATIONS’ ACTIVITY TO WORK AROUND THE ERROR AND SUBMIT THE PROPOSAL (see Figure 3).
For proposals that require the PHS Human Subjects and Clinical Trials Information V3.0 form, please prepare and submit via Grants.gov Workspace or NIH ASSIST.
Figure 2. Error Message
 PLEASE NOTE: This issue does not impact submissions that do not include a Study Record on the PHS Human Subjects and Clinical Trials Information V3.0 form.
The PERIS™ team is working with our vendor to resolve this issue as quickly as possible and will alert users once this has been corrected. Thank you for your patience.
PLEASE NOTE: This issue does not impact submissions that do not include a Study Record on the PHS Human Subjects and Clinical Trials Information V3.0 form.
The PERIS™ team is working with our vendor to resolve this issue as quickly as possible and will alert users once this has been corrected. Thank you for your patience.News Categories
